The Promise of mRNA Vaccines
A worldwide effort is underway to develop effective vaccines for the SARS-CoV-2 virus. After 30 years of development, will the promise of engineered mRNAs rise to the challenge?
The world has been shaken by a small strand of viral RNA, encoding proteins with the single purpose of viral replication and transmission. As nonliving bits of coated RNA or DNA, viruses are not vulnerable to traditional antibiotics. Damage to the host often results from the destruction of host cells during viral replication or via the immune response itself. The severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) partners a highly contagious virus with significant mortality rates. The current pandemic has mobilized a worldwide effort to develop therapeutics and vaccines for SARS-CoV-2 and has invigorated efforts to develop mRNA vaccines for humans.
Viral epidemics and pandemics offer devastating examples of lethal infectious disease. These include the most severe influenza pandemics of 1918, 1956, and 1968 (20 to 50 million influenza deaths, 1918–20), the human immunodeficiency virus pandemic (HIV, 38 million deaths, 1981 to present), and the smallpox virus, which appeared in regular epidemics reaching back at least 3,500 years. During the 20th century alone, smallpox is estimated to have killed 300 million people worldwide. Smallpox (“small pockes,” family Poxviridae) was highly contagious, displayed an approximately 30 percent mortality rate, and was uniformly feared. It was with smallpox that prior exposure to the pathogen was first widely recognized as protective from subsequent infection.
Dating back to the 17th century, the practice of introducing pus material from a smallpox victim into subcutaneous tissue of an unexposed person (variolation), was first practiced. Using even a tiny dose of weakened smallpox virus to provide protection was risky. Variolation treatment sometimes created a full case of smallpox and some patients died from the resulting disease.
In 1798 the British physician Edward Jenner famously published an alternative to variolation. He substituted cowpox, a poxvirus that causes mild disease in humans, in the inoculation procedure and named the process “vaccination” (vacca, Latin for “cow”). He first vaccinated an 8-year-old boy who then displayed resistance to smallpox exposure. In 1979 smallpox itself was finally eradicated from human populations via a vaccination program managed by the World Health Organization. While strategies to stimulate the immune system with a viral mimic have matured dramatically in the past 222 years, using weakened live virus or purified viral proteins to train the immune system continues to be our most effective weapon against viral disease.
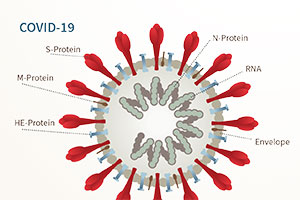 Vaccinology in Brief
Vaccinology in Brief
A successful vaccine confers immunological resistance to a specified viral or bacterial pathogen. Stimulated by the identification of foreign viral components, virus-specific antibody levels and responsive immune system cell types rise during an immune response. Antibodies can drive an immune response to destroy infected cells or may directly neutralize viral particles. Effective vaccine components will be antigenic, meaning readily recognized by antibodies, and are usually proteins found on the surface/envelope of the virus. These isolated proteins are not capable of causing disease, but merely train the immune system to recognize the intact virion.
One challenge of vaccine development is identifying effective viral antigens and producing them correctly in the laboratory. Proteins are like strands of beads that are folded into a particular three-dimensional structure. The folded structure must be precisely duplicated for a vaccine to correctly represent intact viruses. Once an antigenic protein is prepared and readied for injection into a host, significant testing for the effectiveness or efficacy must be performed in animal models and, eventually, in humans.
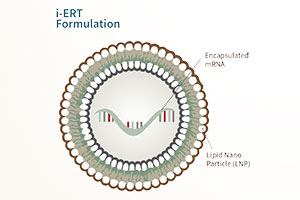 DNA/RNA Vaccine Design
DNA/RNA Vaccine Design
The synthesis of protein in any living cell begins with information flow from the DNA of a gene, which is transcribed to messenger RNAs (mRNA) and subsequently translated as the mRNA “read” by cellular enzymes to produce the encoded protein. For the past 30 years, scientists have been developing the technology to use DNA or mRNA molecules as a vaccine that causes the patient to produce viral antigens in their own cells. Such a vaccine would enable the host cells to produce correctly folded and processed proteins as an exact representation of viral proteins, but without causing disease. In addition to the benefits of synthesizing the protein in the host cells, DNA and mRNA strategies are rapidly scalable for the production of millions of doses of vaccine in a relatively short period of time.
DNA Vaccines
DNA vaccines consist of an engineered DNA sequence delivered to the cells primarily via direct injection or as an innocuous virus. Though DNA vaccines have shown clear promise, they have not been highly effective in generating a strong response from the immune system. Several vaccinations are needed to achieve significant levels of immune system response. Furthermore, there is the potential for viral DNA to integrate into the host chromosome, potentially causing detrimental mutations (Li et al., 2012). Though no DNA vaccines are approved for human use at this point, the past five to seven years have shown improvements in vaccine effectiveness in early clinical trials and animal studies. DNA vaccines against Zika virus, HIV, Ebola virus, influenza virus, and now SARS-CoV-2 are in development (Ebony and Weiner, 2020).
mRNA Vaccines
mRNA vaccines are simple lipid nanoparticles mingled with mRNA strands that encode antigenic viral proteins. The lipids enable transfer of the mRNA across the cell membrane and the rapid production of viral proteins. Recent advancements in the computational design of mRNA vaccines and the purification of the synthetic RNA have brought this strategy to the forefront. A particularly exciting advancement in mRNA vaccines is the inclusion of a second mRNA in the nanoparticles. This extra gene encodes a protein enzyme that makes copies of the mRNAs once inside the host cell. The mRNAs are amplified dramatically in a recipient cell, generating a much higher level of protein production and immune system stimulation. Accordingly, small doses of an mRNA vaccine can generate a strong response by the immune system.
This approach has been shown to stimulate robust antibody production and to activate immune cells associated with long-lasting immunity (Pardi et al., 2018). mRNA vaccines are already approved for animal use and have shown success in eliciting an immune response with HIV, Zika virus, Ebola virus, and influenza virus in animal trials (Zhang et al., 2019). The SARS-CoV-2 mRNA vaccines, if approved, would be the first to gain approval for use for vaccination of humans.
The Advantages of Synthetic mRNA Vaccines
In the context of emerging infectious disease, mRNA vaccines convey several promising characteristics. mRNA vaccines elicit a potent immune response in CD8+T cells, responsible for detecting viral infection. Additionally, unlike protein vaccines, mRNA vaccines remain stable and highly effective for long periods in the absence of refrigeration, even as long as six months in 40 degrees Celsius (Zhang et al., 2019). This enables the wide distribution of vaccines, especially in poorer tropical areas without readily available cooling methods. mRNA vaccines can also be engineered to protect against a variety of similar viruses rather than only one virus, as shown in animal applications.
The capacity to rapidly mobilize mRNA vaccines was famously displayed at the beginning of this pandemic. The DNA sequence of SARS-CoV-2 was first shared by Chinese researchers on January 7, 2020. Over the next 42 days, research teams at Moderna analyzed the sequence data, identified a “spike” protein on the surface of the virus as an antigenic vaccine candidate, designed and produced the mRNA vaccine, and submitted vaccine mRNA-1273 for Phase 1 clinical trials on February 24, 2020. Clinical trials have been moving forward rapidly and showing promise, though no full data sets have been released at this point.
In an interview with STAT, Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, noted that Moderna’s Phase 1 data clearly showed “antibody that was neutralizing live virus at levels that you would predict would be protective,” prompting his cautious optimism. At least two other mRNA vaccines, produced by BioNTech/Pfizer (New York, New York) and CureVac (Tübingen, Germany). are moving quickly into trials with human subjects.
Pushing the Pace
The mobilization of a worldwide effort to develop SARS-CoV-2 vaccines defines an unprecedented moment of scientific cooperation and singular focus. Private pharmaceutical companies are joining in partnerships, and governments are investing deeply in facilitating the production and clinical evaluation of vaccine candidates. Critical questions regarding the safety and efficacy of vaccine candidates have to be addressed before approvals will be gained. The characteristics of a protective immune response are not yet defined for SARS- CoV-2. If the vaccine proves protective, the durability of the protection is unpredictable. This pandemic has shown higher rates of transmission and mortality in distinct human populations (age, ethnicity, health factors), and the effectiveness of vaccines for those groups needs to be examined.
In the United States, the National Institutes of Health has announced the establishment of the ACTIV collaborative program (Accelerating COVID-19 Therapeutic Interventions and Vaccines). This program was developed to streamline the process of evaluating candidate vaccine safety and efficacy. ACTIV has the capacity to analyze vaccines in parallel while ensuring properly controlled and randomized efficacy trials (Corey et al., 2020). Similar partnerships are rising up around the world.
The need for a robust pandemic response from the biomedical research community has been met with encouraging governmental funding and partnerships among private companies, which have dramatically increased the pace of testing and approval. Approximately 150 vaccine candidates against SARS-CoV-2 are in different phases of development at this time. May this work improve our ability to respond to new pathogens, protect populations around the world from the current pandemic, and restore our ability to live, work, and educate in community.
 Grace Lank (’20)
Grace Lank (’20)
Grace Lank is a 2020 alumna of Pepperdine University, having recently graduated with a bachelor of science in biology. As an undergraduate she conducted research with the Darrow Stem Cell Institute, investigating the efficacy of stem cell injections on pain reduction. During her studies at Pepperdine, she became increasingly interested in the biochemistry of viruses and vaccines and spent time working with AIDS patients at a clinic in her hometown of Seattle, Washington. Motivated by her time working in a clinical setting and a desire to contribute to the study and treatment of HIV, she pursued and was awarded a post-baccalaureate fellowship with the National Institute of Allergy and Infectious Diseases (NIAID) in Bethesda, Maryland. She has recently joined the research group of Anthony Fauci, director of NIAID.
 Jay Brewster
Jay Brewster
Jay Brewster is a professor of biology at Seaver College and serves as the divisional dean of the Natural Science Division. He joined Pepperdine in 1997 after earning his PhD from Rice University (Department of Biochemistry and Cell Biology) and serving as a research associate at the McLaughlin Biomedical Research Institute. His research at Pepperdine focuses on cellular signaling dynamics in response to nanoparticulate pollutants.
References
- Corey L, Mascola JR, Fauci AS, and FS Collins (2020), “A strategic approach to COVID-19 vaccine R&D,” Science, Vol 368:6494, 948–50.
- Ebony GB, and DB Weiner (2020), “DNA vaccines: prime time is now,” Current Opinion in Immunology, Vol 65, 21–27.
- Li L, Saade F, and N Petrovsky (2012), “The future of human DNA vaccines,” Journal of Biotechnology, Vol 162, 171–182.
- Pardi N, Hogan MJ, Porter FW, and D Weissman (2018), “mRNA vaccines - a new era in vaccinology,” Nature Reviews: Drug Discovery, Vol 17, 261–279. Zhang C, Maruggi G, Shan H, and J Li (2019), “Advances in mRNA vaccines for infectious diseases,” Frontiers in Immunology, Vol 10:594, 1–13.
